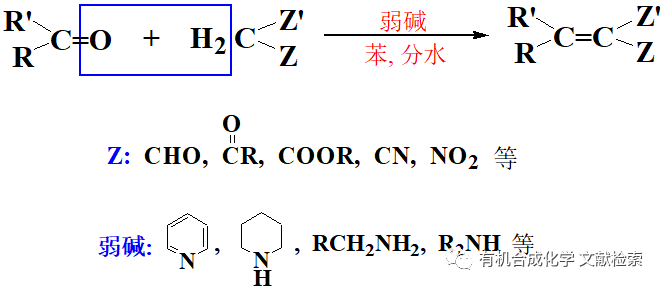
NO2Cl(硝酰氯)以前是由發煙硝酸和氯磺酸制得,由于這個方法不方便以及危險性大,另一個替代方法是 NaNO3/TMSCl/AlCl3體系【Olah, G. A; Ramaiah, G, Synthesis,1994, 468.】。NaNO3 和TMSCl 反應生成硝酰氯,然后再AlCl3 的作用下經過類似F-C反應的親電取代過程得到硝化物, NaNO3也可用KNO3代替。這個硝化反應條件很溫和,選擇性很好,如果用其他硝化方法得到的選擇性差,不妨試試這個硝化條件,其缺點是有時轉化率不高。
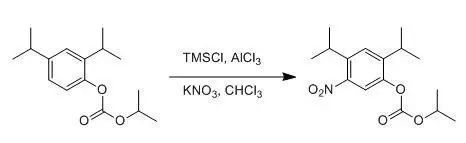
To a suspension of KNO3 (60.6 g, 0.6 mol) in CHCl3 (500 mL) wasadded TMSCl (102 mL, 0.8mol) and carbonic acid 2,4-diisopropyl-phenyl esterisopropyl ester (105.6 g,0.4 mol) successively at 0oC, the mixture was stirred for 0.5hour. Then AlCl3 (160 g, 1.2 mol) was added in one portionat 0 oC. The mixture wasstirred for another 2 hours. TLC showedmost of the starting material was consumed and the product was present. The resulting mixture was poured intoice-water, extracted with CHCl3. The combined organic layers were washed with Sat.NaHCO3solution, brine successively, dried over Na2SO4 andevaporated under reduced pressure to give the crude product as an oil. To thecrude product was added 100 mL petroleum ether, the desired compound wasrecrystallized from the ether in the bath of dry ice-acetone, which wasfiltered and washed with petroleum ether to give the desired product as a paleyellow solid (27.2 g,22%).
硝酸酯,包括BuONO2/Nafion-H,MeONO2/BF3, Me3SiONO2等,作為硝化試劑,能使活化的苯環發生硝化。
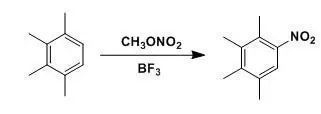
Asolutuion of 1, 2, 3, 4-tetramethylbenzene (1.34 g, 10 mmol) in nitromethane (20 mL) was addedmethyl nitrate (2.31 g,30 mmol). The mixture was stirred at 25oCand saturated with boron trifluoride gas. The mixture was stirred for 1 h then washedwith Sat.NaHCO3 solution, extracted with CH2Cl2. The combined organic layers were evaporatedunder reduced pressure, the residue was purified by column chromatography togive the product (1.62 g,90%).
【Olah, G. A; Lin, H.C. Synthesis, 1973, 488.】
本文內容非原創,版權歸原作者所有。