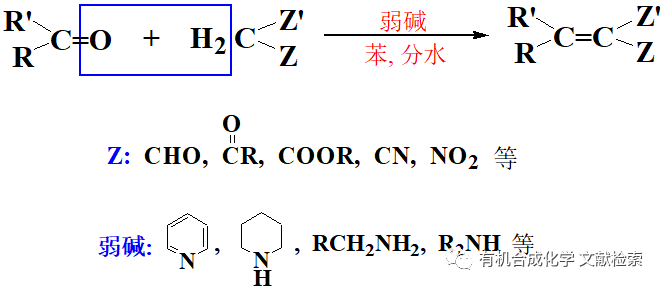
胺先與氯甲酸酯反應(yīng)得到相應(yīng)的R氧基碳酰胺,然后再與另一分子胺反應(yīng)生成脲。本方法適用范圍也很廣,對(duì)那些底物很昂貴、或較難得到的,本方法尤為適用。一般來(lái)說(shuō)比較常用的為氯甲酸對(duì)硝基苯酯和氯甲酸苯酯, 另外還有氯甲酸2-異丙烯酯,氯甲酸2-三氟乙基酯或氯甲酸2-三氯乙基酯等等。
下面以氯甲酸對(duì)硝基苯酯為例作為介紹:
氯甲酸對(duì)硝基苯酯主要用于伯胺的反應(yīng),其反應(yīng)機(jī)理是中間體對(duì)硝基苯氧基碳酰胺在堿性條件下,脫去對(duì)硝基苯酚得到相應(yīng)的異氰酸酯,然后再與另一分子胺反應(yīng)得到脲。使用本方法一個(gè)主要的注意點(diǎn)是第一步對(duì)硝基苯氧基碳酰胺的制備,一定要選擇好相應(yīng)的堿,用好當(dāng)量。另外也有文獻(xiàn)在第一步用過(guò)量的堿生成異氰酸酯的溶液,馬上再與另一分子胺反應(yīng)。該法的一個(gè)缺點(diǎn)就是有時(shí)產(chǎn)生的黃色的副產(chǎn)物對(duì)硝基苯酚,不易除干凈(一般用強(qiáng)堿洗)。
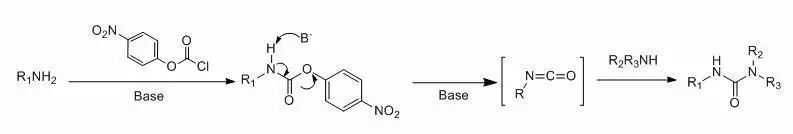
氯甲酸對(duì)硝基苯酯也可以與仲胺反應(yīng)生成脲,一般在DMAP-CH3CN,加熱體系進(jìn)行胺交換。
1、芳香伯胺的對(duì)硝基苯氧基碳酰胺和脲的合成示例 【WO2004/9558 A2 (2004/01/29)】
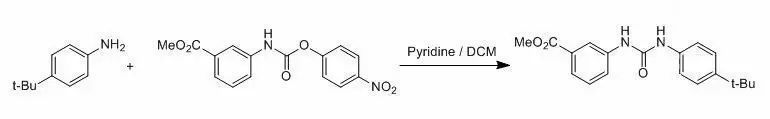
To a solution of methyl 3-aminobenzoate (1.0g, 6.5 mmol) and pyridine (1.0 mL) in 100 mL of dichloromethane was added asolution of 4- nitrophenylchloroformate (1.4 g, 6.7 mmol) in 10 mL of dichloromethanedropwise at 0oC under N2 atmosphere. The resultingmixture was stirred at r.t. for 20 h before poured into ice-water. The mixture was extracted withDCM (3 x 100 mL). The combined organic phases were washed with 0.5 Naq. HC1 andbrine, dried over anhydrous Na2SO4 and filtered. Thefiltrate was concentrated to give the crude product, which was purified bycolumn to afford 2.1 g of 3-[3-(4-tert-Butyl-phenyl)-ureido]-benzoic acidmethyl ester (94 %)
2、脂肪伯胺的對(duì)硝基苯氧基碳酰胺和脲的合成示例 (J. Org. Chem. 2004, 46-53)
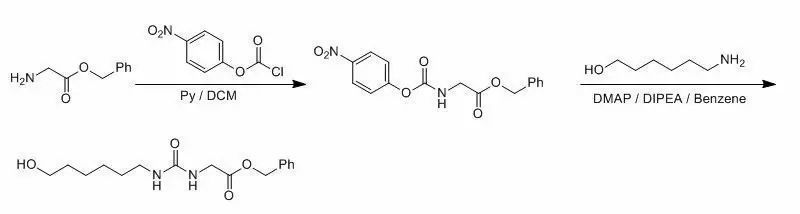
To a solution of 4-nitrophenyl chloroformate(2.3 g, 11 mmol) in 20 mL of CH2Cl2 was added a solution of benzyl glycinate (0.62 g, 3.8mmol) in 4 mL of 1:1 CH2Cl2/ pyridine at 0 °C dropwise. The solution was stirred for30 min and then diluted with 100 mL of CH2Cl2. The reaction mixture was washedwith 1 M NaHSO4 (3 x 50 mL) and brine (3 x 50 mL). The organicphase was concentrated to the crude product, which was purified by columnchromatography to afford 0.81 g of N-(4-nitrophenyloxycarbonyl)benzyl glycinate(65%) as white solid. To thesolution of N-(4-nitrophenyloxycarbonyl)benzyl glycinate (0.79 g,2.4 mmol) in 10 mL of benzene were added 1,6-aminohexanol (0.34 g, 2.9 mmol),DMAP (88 mg, 0.72 mmol) and diisopropylethylamine (0.46 g, 3.6 mmol) at roomtemperature. The reaction mixture was stirred at for 30 min before diluted with50 mL of CH2Cl2. The mixture was washed with 1 M NaHSO4 (3 x 50 mL), 2% Na2CO3 (3 x 50 mL) and brine (3 x50 mL). The organic phase was dried and concentrated to the crude product,which was purified by column chromatography to afford 0.61 g of [3-(6-hydroxyhexyl)ureido]aceticacid benzyl Ester (86%) as white solid..
3、利用氯甲酸對(duì)硝基苯酯一鍋法合成脲示例(J. Med. Chem. 2001, 1021-1024)
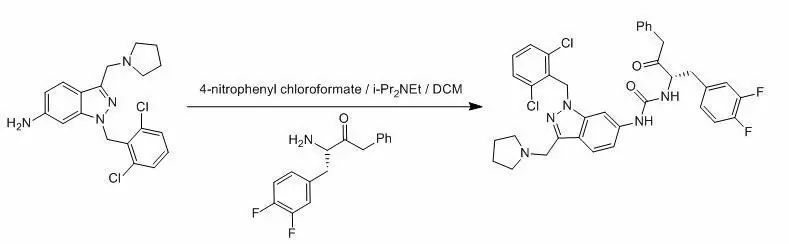
To a solution of 1-(2,6-dichloro-benzyl)-3-pyrrolidin-1-ylmethyl-1H-indazol-6-ylamine(374 mg, 1.0 mmol) and diisopropylethylamine (640 mg, 5.0 mmol) in 100 mL ofDCM was added a solution of 4-nitrophenyl chloroformate (220 mg, 1.1 mmol) in10 mL of DCM at –20 oC under N2 atmosphere. The resultingmixture was stirred for 30 min and then added 3-amino-4-(3,4-difluoro-phenyl)-1-phenyl-butan-2-one(275 mg, 1.0 mmol). After stirred at – 20 oC for 30 min, the mixturewas warmed to room temperature and then stirred for another 6 h before pouredinto water. The reaction mixture was extractedwith DCM (3 x 100 mL). The combined organic phases were washed with brine (3 x50 mL), dried over anhydrous Na2SO4 and filtered. Thefiltrate was concentrated to the crude product, which was purified by columnchromatography to afford 175 mg of 1-[1-(2,6-dichloro-benzyl)-3-pyrrolidin-1-ylmethyl-1H-indazol-6-yl]-3-[1-(3,4-difluoro-benzyl)-2-oxo-3-phenyl-propyl]-urea (26 %)
4、氯甲酸對(duì)硝基苯酯用于仲胺的脲合成示例 (J. Med. Chem. 1999, 5254-5265)
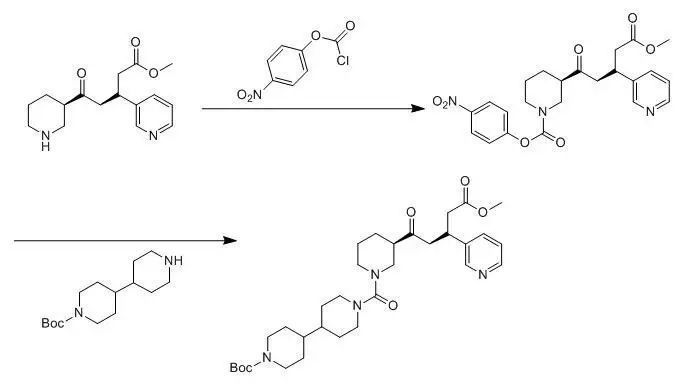
To a solution of 5-oxo-5-piperidin-3-yl-3-pyridin-3-yl-pentanoicacid methyl ester (2.9 g, 10 mmol) in DCM (200 mL) wasadded 4-nitrophenylchloroformate (2.0 g, 10 mmol) and NMM (6.0 mL, 30 mmol) at0 oC. The resulting mixture was stirred for 2 h before poured intowater (15 mL). After separated, the organic layer was dried over anhydrous Na2SO4 and evaporatedto oil, which was dissolved in 100 mL of MeCN. The solution was then treated by[4,4']bipiperidinyl-1-carboxylic acid tert-butyl ester (4.3 g, 15 mmol) and DMAP(1.2 g, 10 mmol), and heated to reflux for 24 h. After removal of the solvent,the residue was dissolved in EtOAc (200 mL). The organic phase was washed with 1N NaOH (3 x 100 mL), brine (3 x 100 mL) and dried over anhydrous Na2SO4. Afterfiltered, the filtrate was concentrated to the crude product, which was purifiedby silica gel chromatography to afford 4.1g of 1'-[3-(4-methoxycarbonyl-3-pyridin-3-yl-butyryl)-piperidine-1-carbonyl]-[4,4']bipiperidinyl-1-carboxylicacid tert-butyl ester (69 %)
本文內(nèi)容來(lái)源于網(wǎng)絡(luò),版權(quán)歸原作者所有。