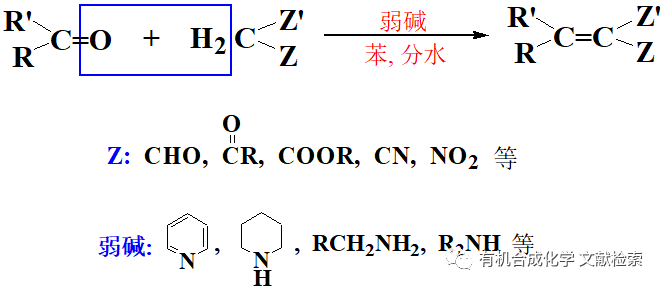
1、Cu 催化下芳香鹵代烴或(TfO- )和K 4 [Fe(CN) 6 ]反應氰基取代
Thornds Schdreind, Alexander zapf 報道了一種在Cu催化下芳香鹵代烴或(TfO-)和K 4 [Fe(CN) 6 ]反應高收率生成氰基化合物的方法。作者經過一系列實驗,使用不同的銅催化劑,以及不同的配體與溶劑,從而得到了最好的實驗條件。即:Cu(BF 4 ) 2 . 6 H 2 O為催化劑,DMEDA為配體,DMAc為溶劑。

No condition details were available in this literature. The general reaction conditions that the author gave were: 2.0 mmol aryl halide, Cu(BF 4 ) 2 .6H2O(0.1 eq), 20 mol% dry K 4 [Fe(CN) 6 ], 2 mL DMAc, 20 mol% KI, 20 mol % Na 2 CO 3 , 100 mol % DMEDA.
2、微波反應芳鹵氰基化
直接取代大多用高溫反應,有人開發了使用微波反應做這一取代。

A dried heavy-walled pyrex tube was charged with organo-bromide (0.2 mmol), Zn(CN) 2 (23.5 mg, 0.2 mmol) and Pd(PPh 3 ) 4 (6.9 mg, 6.0 ímol) in DMF (1 ml). The reaction mixture was flushed with nitrogen and the screw cap tightened thoroughly before mixing with a Whirlimixer. The reaction mixture was exposed to microwave irradiation (60 W) for 2 min (for 2g 2.5 min). The reaction tube was allowed to reach room temperature before the reaction mixture was diluted in EtOAc (60 mL) and washed with water. The organic phase was dried and the solvent was removed under reduced pressure. The crude product was purified by column chromatography to give the pure nitrile.