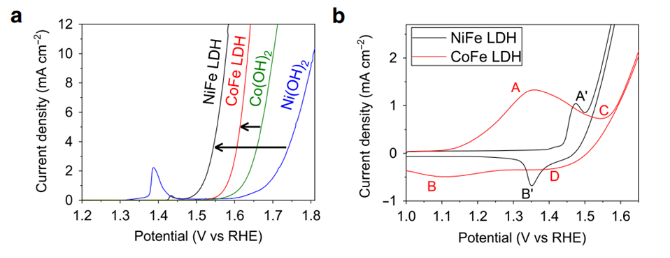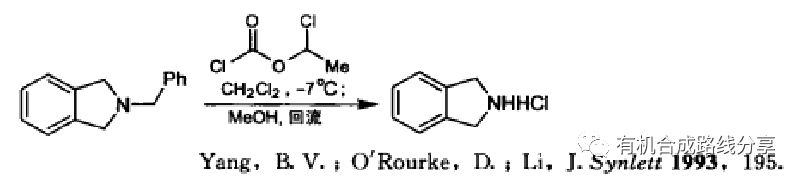
Takemoto手性催化劑是由手性二胺和苯胺制備得到的手性硫脲。Takemoto課題組,在2003年報道了此類催化劑,此類催化劑對含有硝基或羰基等強吸電子基團的烯烴進行不對稱Michael加成反應,具有較高的選擇性和產率。

【T. Okino, Y. Hoashi, T. Fukukawa, X. Xu, Y. Takemoto, J. Am. Chem. Soc., 2005, 7, 119-125】
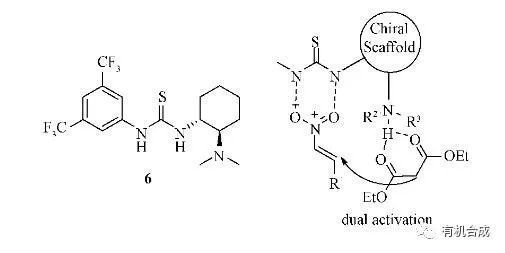
反應機理
在手性框架下對親核碳負離子化合物和親電共軛體系進行同時活化。
反應操作
催化劑的制備
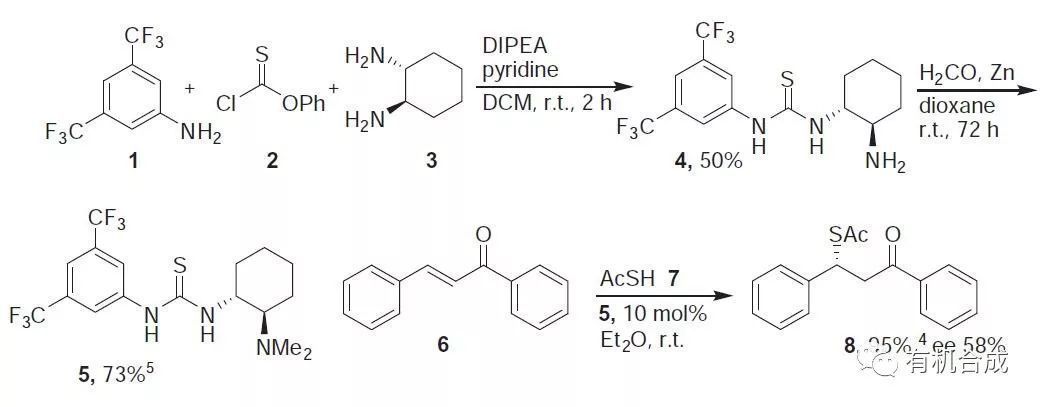
1-[3,5-Bis(trifluoromethyl)phenyl]-3-[(1R,2R)-2-(amino)cyclohexyl]thiourea (4).
To 1 (280 mL,410 mg, 1.82 mmol) and pyridine (160 mL, 157 mg, 1.99 mmol) in DCM (6 mL) was added phenyl chlorothioformate 2 (250 mL, 312 mg, 1.81 mmol) at r.t. and the mixture was stirred for 2 h. To this was added dropwise (1R,2R)-1,2-diaminocyclohexane 3 (207 mg, 1.81 mmol) in DCM (1 mL) and DIPEA (300 mL, 234 mg, 1.81 mmol). After 15 min, the mixture was refluxed and sat NaHCO3 (7 mL) was added. The aq phase was extracted with DCM and the combined organic layers were worked up and chromatographed (CHCl3:MeOH, 7:1) to afford 4 (50%), mp 69 ℃.
Methylation of (4). Zn powder (102 mg, 1.56 mmol), AcOH (180 mL, 187 mg, 3.12 mmol), and aq formaldehyde (37%, 95 mL, 1.17 mmol) were added to 4 (150 mg, 389 mmol) in dioxane (0.5 mL) at r.t. After the mixture was stirred for 72 h, NH4OH (500 mL) was added and the aq phase was extracted with DCM. Usual workup and chromatography (CHCl3:MeOH, 7:1) afforded 117 mg (73%) of solid 5.
【Berkessel A, Synthesis, 2009, 2113】
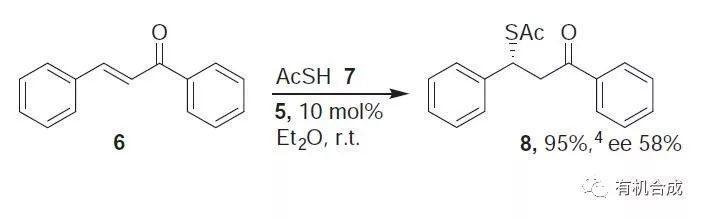
Ketothioester (8). To a stirred mixture of chalcone 6 (0.1 mmol) and thiourea 5 (10 mol %) in ether (0.5 mL) was added thioacetic acid (0.2 mmol) and after 3 h stirring at r.t., chromatography (silica gel, EA/hexane1:20–1:3) gave 8 (95%, 58% ee).
【Wang W, Tet Lett., 2006, 47, 3145】
相關文獻
1* Takemoto Y J Am Chem Soc 2003 125 12672
2* Takemoto Y J Am Chem Soc 2005 127 119
3* Berkessel A Angew Chem Int 2005 44 7466
4* Wang W Tet Lett 2006 47 3145
5* Berkessel A Synthesis 2009 2113
6* Takemoto Y Chem Pharm Bull 2010 58 593
編譯自:Organic Syntheses Based On Name Reactions, 3RdEd, A. Hassner, Page 479.