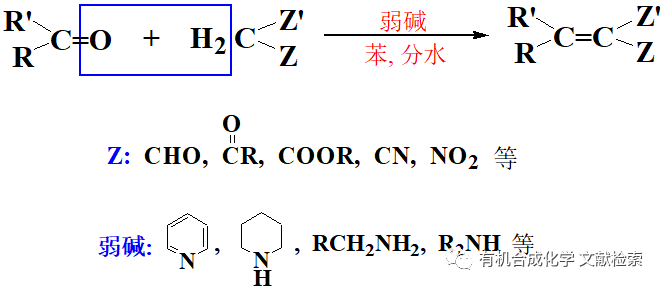
化學(xué)化學(xué)方法將醛或酮的羰基直接轉(zhuǎn)化為亞甲基有如下幾種:1) Clemmensen 還原;2) Wolff-Kishner-黃鳴龍還原; 3) LiAlH 4 -AlCl3 法; 4) NaBH 4 -CF3CO2H 法; 5) Et3SiH-BF3 or CF3CO2H法; 6) HI-Phosphorus 法;7) 催化氫化法 .
催化氫化轉(zhuǎn)化羰基為亞甲基由Brieger報(bào)導(dǎo),他們使用Pd/C作為催化劑,并用FeCl 3 作Lewis酸促進(jìn)劑. 另外,有綜述專門提到用甲酸銨作催化氫化轉(zhuǎn)移劑.
近期,哈佛的Andrew Myers 在其關(guān)于腙衍生物工作中報(bào)導(dǎo)了用Sc(OTf) 3 作催化劑的有效的低溫Wolff-Kishner還原.
對于有些結(jié)構(gòu)復(fù)雜,帶有多種敏感官能團(tuán)時(shí),以上這些一步或一鍋法無法將醛或酮的羰基直接轉(zhuǎn)化為亞甲基,因此可能需要將醛或酮轉(zhuǎn)化為其他官能團(tuán)進(jìn)行除去。較為常見的方法是轉(zhuǎn)化為醇羥基除去(醇羥基除去方法見脫羥基反應(yīng)部分);另外,可以將醛或酮轉(zhuǎn)化為乙二硫醇的縮醛或酮,再用 Raney Ni 氫化還原為亞甲基;另外也有文獻(xiàn)將醛或酮轉(zhuǎn)化為對甲苯磺酰肼的腙,再用 DiBAL 或 NaBH(OAc)3 還原。

Clemmensen 還原
將醛或酮用鋅汞齊處理在濃鹽酸下加熱可以將醛或酮的羰基轉(zhuǎn)化為亞甲基. 這就是克萊門森(Clemmensen)還原 . 很明顯,對酸敏感的底物(醛酮)不能使用此法還原(如醇羥基、C=C等)。

此法對于還原芳香酮和雙烷基酮有一定的通用性。其用在還原芳香酮是間接在芳環(huán)上引入直鏈烴基的方法之一。

反應(yīng)機(jī)理
反應(yīng)發(fā)生在鋅催化劑表面. 由于將相應(yīng)的醇用于此反應(yīng)條件并不能得到亞甲基化合物,因此該反應(yīng)中間態(tài)可能沒有醇產(chǎn)生. 下圖大致描述了克萊門森(Clemmensen)還原的機(jī)理.

Clemmensen 還原芳基酮示例

Amalgamated zinc is prepared by shaking for five minutes a mixture of 120 g. of mossy zinc,12 g of mercuric chloride, 200 mL of water, and 5–6 mL of concentrated hydrochloric acid contained in a 1-L round-bottomed flask. The solution is decanted and the following reagents are added, in the order named, to the zinc: 75 mL of water, 175 mL of concentrated hydrochloric acid, 100 mL of toluene, and 50 g (0.28 mol) of β-benzoylpropionic acid. The flask is fitted with a vertical condenser connected to a gas absorption trap, and the reaction mixture is boiled vigorously for twenty-five to thirty hours. Three 50 mL portions of concentrated hydrochloric acid are added at approximately six-hour intervals during the refluxing period. After cooling to room temperature the layers are separated. The aqueous layer is diluted with 200 mL of water and extracted with three 75 mL portions of ether. The toluene layer and the ether extracts are combined, washed with water, and dried over calcium chloride. The solvents are removed by distillation under reduced pressure on the steam bath, after which the χ-phenylbutyric acid is distilled at 178–181°/19 mm. (148–154°/8–10 mm., 125–130°/3 mm.). The yield of acid, which melts at 46–48°, is 38–41 g. (82–89%).
Clemmensen 還原二烷基酮示例

Clemmensen reduction of methyl 8,8,8-trifluoro-4-ketooctanoate was carried out in a 2-liter, three neck, round-bottom flask fitted with a Liebig condenser surmounted by a Friedrichs condenser, a Hershberg stirrer with a Trubore bearing, and a 50-mL dropping-funnel. The condensers were sealed with a Dry-Ice trap, and a gas washing bottle filled with petroleum ether (65-110 o C). The flask was charged with 300 g. of amalgamated, reagent grade, mossy zinc, 100 mL of water, 240 mL of concentrated hydrochloric acid, and 93.0 g (0.41 mole) of methyl 8,8,8-trifluoro-ketooctanoate. The mixture was heated with an oil-bath at 120-130℃. The reactiori was continued for 112 hours, and an additional 50 g. of amalgamated zinc and 50 mL of concentrated hydrochloric acid were added every 24 hours. The reaction mixture was extracted with four portions of isooctane, and then the extract was washed with water and dried by azeotropic distillation with ethylene chloride. Fractional distillation of the residue through a Vigreux partial take-off, fractionating column yielded two main fractions: ω-trifluorooctanoic acid, 31.6 g. (72%, converted keto acid), b.p. 129 o C (10 mm.);and 8,8,8-trifluoro-4-ketooctanoic acid, 36.0 g., b.p. 136 ℃