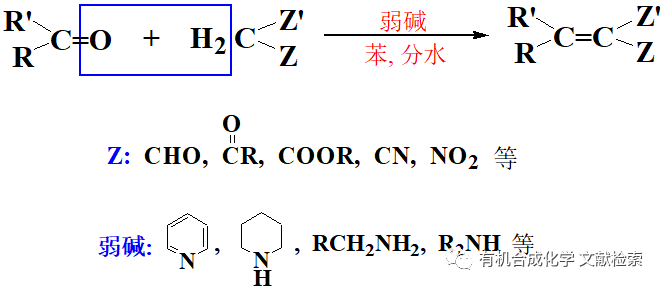
α-氨基酸經重氮化后進行分子內的取代反應離去氮氣,接著再進行一次取代反應得到構型保持的α取代羧酸的反應。反應體系中有F, Cl,Br,水都可以進行此反應。反應通常以水作為溶劑,在酸性體系中進行。由于鹵離子在酸性條件下,比水分子的親核性強,因此當體系中有鹵離子(F, Cl,Br)時,得到的是α-鹵代羧酸,否則是α-羥基酸。
反應機理
重氮化后由于分子內的鄰助效應,先進行一次分子內取代,形成的不穩定的中間體,又很快進行一次分子間的取代反應構型保持。

反應實例

D-alanine (8.9 g, 0.1 mol) and KBr (11.9 g, 0.1 mol) was dissolved in 100 ml, of a 30percent HBr water solution and kept in ?15°C dry ice/ethylene glycol bath. NaNO2 (10.35 g, 0.15 mmol) were dissolved in 15 ml water and slowly dripped in the above solution under argon atmosphere. The reaction was allowed to proceed for 3 hr and to warm from ?15°C to room temperature. It was then put under vacuum for 30 min. Product was extracted by diethyl ether (25 ml × 3). Organic phases were combined and dried over Na2SO4. Then the solvent was evaporated under vacuum, and the crude product was further purified by distillation at 115°C, high vacuum. The pure product was obtained as a colorless oil in 82percent yield.
【Gao, Yu; Kodadek, Thomas; Chemistry and Biology; vol. 20; nb. 3; (2013); p. 360 - 369】

L-Threonine (25.0 g, 210 mmol) and potassium bromide (87.4 g, 73.4 mmol) were dissolved in sulfuric acid (2.5 M, 500 mL) followed by cooling it down to 0 °C under stirring. A mixture of sodium nitrite (22.0 g, 32.8 mmol) in water (100 mL) was added dropwise over 3 h at 0 °C.The obtained solution was stirred for 12 h at room temperature and then extracted with Et2O (3 × 250 mL). The combined organic layers were washed with saturated brine ( 2 × 250 mL),dried over MgSO4, filtered and evaporated under reduced pressure to obtain the product 6 as a yellow oil. Yield: 90percent (34.4 g).
【Kanlidere, Zeynep; Jochim, Oleg; Cal, Marta; Diederichsen, Ulf; Beilstein Journal of Organic Chemistry; vol. 12; (2016); p.2136 - 2144】

General procedure: The (S)-amino acids (10mmol, 1eq) were dissolved in HCl (26mL of 13M) and temperature of solution was maintained below ?5oC. The chilled aq. NaNO2 (20mmol, 2.0eq) was added as drops to this solution and temperature was controlled below 0°C and stirred for 5h in ice bath. The ice bath was removed and the solution was stirred overnight, extracted with
EtOAc (3×25mL) at highly acidic pH. The organic extract was concentrated which afforded yellow oily products 12a–d which was further concentrated under N2.4.4.3 (2S)-2-Chloro-4-methylpentanoic acid 12c Colorless oil (0.77 mL, 0.83 g, 80percent)
【Saddiqa, Aisha; Raza, Abdul R.; Black, David Stc.; Kumar, Naresh; Tetrahedron Asymmetry; vol. 25; nb. 9; (2014); p. 736 -743】

In a 3-necked 1000 ml. RB flask, sodium nitrite (68.4 g, 0.99 mol) was added in small batches to the aqueous solution of a mixture of DL-serine (52.4 g, 0.50 mol), potassium chloride (130.4 g, 1.75 mol) and HCl (116.0 g of 36.5percent-38percent w/v aq. sol., 1.21 mol) (taken together in 490 mL of water) at 0° C.-10° C. After complete addition, reaction mixture brought to room temperature and kept overnight for the reaction. Solution turned from clear and off-white to clear and light green. The product was salted out with NaCl and extracted with 5 times of 100 mL of ethyl acetate. Organic phase was washed 5 times with saturated NaCl aqueous solution (50 mL each) and then dried over anhy. Na2SO4. Solution was filtered and solvent was evaporated by trap-to-trap distillation method followed by drying in the vacuum chamber.
Product was recrystallized in CH2Cl2. Yield=36.5 g (58percent).
【US8524942; (2013); (B2) English】

To a solution of compound 20 (200 mg, 1 mmol) in 5 mL of pyridinium poly(hydrogen fluoride) is slowly added, with good stirring, sodium nitrite (105 mg, 1.5 mmol). After being stirred at room temperature for 18 h, the water (20 mL) is added. The resulted solution is extracted with ether. The ether layer is washed with 1N NaOH. The aqueous is acetified with HCl and extracted with ether. The ether layer is dried (Na2SO4). The solvent is removed in vacuo.
The crude product 22 (is used for next step without further purification).
【US2011/65724; (2011); (A1) English】

L-isoleucine (10.0 g, 76.2 mmol) was dissolved in 1 M H2SO4 (200 mL) and the solution cooled to ~0 °C using an ice-water bath. Sodium nitrite (42.1 g, 610 mmol) was dissolved in water (200 mL) and then slowly added to the cold stirred amino acid solution over 4 hours. The reaction was allowed to warm to ambient temperature and the mixture continued to stir for 18 h. The aqueous solution was then saturated with sodium chloride, transferred to a separation funnel and extracted with EtOAc (3×75 mL). The combined organic layers were washed with water (2 × 20 mL) and brine (2×20 mL), dried over anhydrous sodium
sulfate. Removal of solvent under reduced pressure gave D in ~99percent (9.97 g)
【Banasik, Brent A.; Wang, Lee; Kanner, Arielle; Mikael Bergdahl; Tetrahedron; vol. 72; nb. 19; (2016); p. 2481 - 2490】

General procedure: The Boc-protected amino acid (1 mmol) was treated with TFA (3 mL) for 15 minutes. Afterevaporation the residue was dissolved in dioxane-water (1:1, 4 mL) and the flask wasplaced in an ice bath. tert-Butylnitrite (0.13 mL, 1.1 mmol) was added and stirring wasmaintained under nitrogen at room temperature for one hour. After pouring the reactionmixture onto celite and evaporation (50 Pa, 30 °C) into a dry free-floating powder,separation was performed utilizing a CombiFlash Rf (Teledyne
ISCO) automated flashchromatography apparatus by means of AcOH-MeOH-EtOAc (1:9:90) on a normal phasesilica column affording the pure a-hydroxy carboxylic acid in the yield specified in Table 1.HO-His(Bom)-OH and HO-Arg(Tos)-OH were isolated by adding water (30 mL) to thereaction mixture followed by freeze drying and HPLC purification.
【Stuhr-Hansen, Nicolai; Padrah, Shahrokh; Str?mgaard, Kristian; Tetrahedron Letters; vol. 55; nb. 30; (2014); p. 4149 -4151】

To a solution of (S)-(4-nitrophenyl) glycine (10 g, 47.6 mmol) in a mixture of water (50 mL), H2SO4 (1M, 60 mL) and acetone (150 mL) at -5° C., was added under stirring, a solution of sodium nitrite (9.85 g, 142.8 mmol) in water (40 mL) dropwise over a period of 30 min. The reaction mixture was stirred at -5 to 0° C. for another 1.5 h, followed by stirring at room temperature for 16 h. Acetone was removed and then the reaction mixture was diluted with 500 mL ethyl acetate. Organic layer was washed with brine, dried over anhydrous Na2SO4, and concentrated. The crude mass was purified by crystallization from
isopropyl acetate (9.0 g, 96percent)
【US2005/277693; (2005);(A1) English】

A solution of (S)-2-aminopent-4-enoic acid (5.0 g,43.4 mmole, 1.0 eq.) in water/acetic acid (100 mE, 8/2 v/v) is added a solution of NaNO2 (7.5 g, 108.5 mmole, 2.5 eq.) in distilled water (20 mE) over 30 minutes period at 0° C. The resulting solution is stirred 2 hours at 0° C., then 12 hours at ambient temperature. The solution is then cooled down to 0° C. again, quenched with a
solution of CH3NH2 in THF (2 M, 18.2 mE, 36.4 mmole, 2.0 eq.). THF is brieflyevaporated under reduced pressure and the residual aqueous solution is acidified with conc. HC1 to pH 2. This acidic solution is extracted with EtOAc (50 mLx4) and the organic layer is combined, dried over Na2SO4, and then concentrated under reduced pressure. The residue is purified by chromatography over silica gel with mixed solvent system of (CH2C12/MeOH/water, 10/5/0.5, v/v/v). The title compound is obtained
in 4.7 g (40.4 mmole, 93percent) as light brown gel
【US2016/333043; (2016); (A1) English】