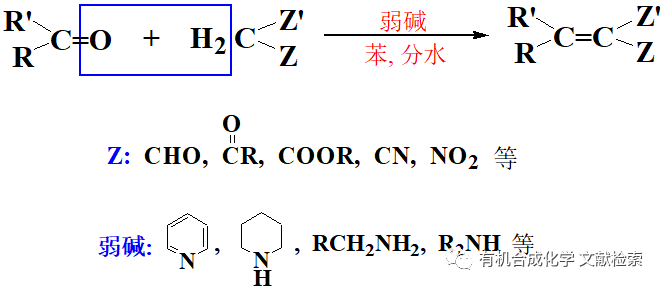
由酯或酰氯經 Weinreb 酰胺合成醛酮
酯和酰氯與有機鋰試劑、格氏試劑反應,產生的酮活性較高繼續反應最終得醇。 如果將酯和酰氯變為相應的 Weinreb 酰胺再與有機鋰試劑、格氏試劑反應則能將反應停止到醛或酮的階段。 

 配合物水解則得到酮。
配合物水解則得到酮。

由 Weinreb 酰胺還原合成醛反應示例 1:

(Benzotriazol-1-yloxy)tris(dimethylamino)phosphonium hexafluorophosphate (BOP.PF 6 ) (163 mg, 0.380 mmol) was added to a stirred solution of N-tert-BOC-(S)-aspartic acid 1-(tert-butyl ester) (5) (100 mg, 0.346 mmol) and triethylamine (54 μL, 0.385 mmol) in dichloromethane (3.5 mL) at room temperature. After 5 minutes of stirring,O,N-dimethylhydroxyamine hydrochloride (40 mg, 0.397 mmol) was added, followed by triethylamine (54 μL, 0.385 mmol). All solid material dissolved within 10 minutes and the mixture was stirred for 2 h at RT. The reaction mixture was washed with 1 M aqueous HCl solution (3 x 1 mL), H 2 O (1 x 1 mL), 1 M aqueous NaHCO 3 solution (2 x 1 mL) and H 2 O (2 x 1 mL). The solvent was removed in vacuo to give a clear yellow oily product, which was purified by flash chromatography on silica, using 30% ethyl acetate/petroleum ether as the eluant, to give the title compound (8) 16 as a colourless oil (100 mg, 86%).
Method A: Triethylsilane (238 μL, 1.485 mmol) was quickly added to a solution of α-tert-butyl β-S-ethyl (S)-N-(tert-butoxycarbonyl) thioaspartate (6) (165 mg, 0.495 mmol) and 10% Pd/C (10.6 mg, 0.0099 mmol) in dichloromethane (2 mL) cooled in an ice bath to maintain the internal temperature between 15-20 °C. The reaction was monitored for 20 minutes, after which the temperature no longer increased. The reaction mixture was then filtered through celite and concentrated in vacuo. Flash chromatography on silica, using 15% ethyl acetate/petroleum ether as the eluant, gave tert-butyl (S)-2-[N-(tert-butoxycarbonyl)amino]-4-oxobutanoate (7) 15 as a clear oil (114 mg, 84%).
Method B: A solution of diisobutylaluminum hydride (DIBAL) in hexane (1 M, 0.55 mL,0.55 mmol ) was added dropwise over 20 minutes to a stirred solution of N-t-BOC-(S)-aspartic acid 1-(tert-butyl ester) N-methoxy-N-methylamide (8) (110 mg, 0.36mmol) in anhydrous THF (1.8 mL) at –78 °C . The mixture was allowed to stir at –78 °C for 2 h. The reaction mixture was partitioned between 0.35 M aqueous NaHSO 4 solution (3.6mL) and diethyl ether (5.5 mL). After separation, the aqueous layer was extracted with diethyl ether (3 x 2 mL). The combined ethereal layers were washed with 1 M HCl solution
(3 x 1mL), 1 M aqueous NaHCO 3 solution (3 x 1 mL), and brine (3 x 1 mL). Concentration in vacuo gave 1-tert-butyl (S)-2-[N-(tert-butoxycarbonyl)amino]-4-oxobutanoate as a colourless oil (92 mg, 95%), which solidified on standing at room temperature.
由 Weinreb 酰胺還原合成酮反應示例2:

To a solution of 3-propenylmagnesium bromide (97 mL of a 0.5 M solution; 48.4 mmol) in THF (15 mL), cooled to 0℃ and under a nitrogen atmosphere, was added dropwise with stirring a solution of tert-butyl 2-(N-methoxy-N-methylcarbamoyl)pyrrolidinecarboxylate (5.0 g, 19.4 mmol) in 15 mL of THF. The mixture was stirred overnight while slowly warming to room temperature. The reaction was quenched by the addition of 80 mL of saturated NH4Cl followed by 50 mL of ethyl acetate and 20 mL of water. The layers were separated, and aqueous layer was extracted with 2 x100 mL of EtOAc. The combined organic layers were dried over MgSO4, filtered, and concentrated, and the crude product was purified on a silica gel column with 10% EtOAc in hexane to obtained the olefin as a clear oil, 4.30 g (88%).