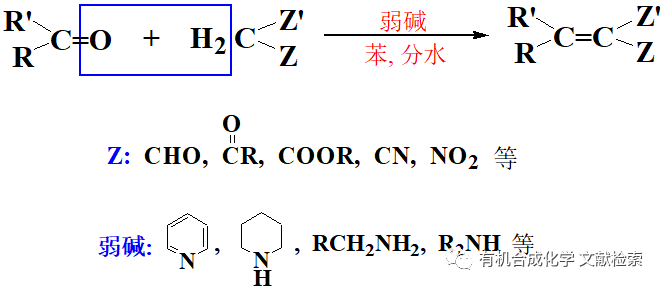
用TosMIC 直接從酮轉化為氰基
對甲苯磺酰甲基異氰(TosMIC) 是有機化學官能團轉換反應中最有用的多功能試劑和合成子之一。TosMIC 在雜環合成中可以用來方便地制備咪唑、噻唑、 惡唑、三氮唑、吲哚、吡咯等。TosMIC 參與的增加一個碳原子的還原氰基化反應和二烷基化反應具有獨到之處。
TosMIC與酮生成腈的還原氰基化反應是該試劑最特征的反應之一。反應的機理是TosMIC在堿的存在下生成碳負離子與酮羰基發生親核加成反應,然后失去磺酰基和甲酰基生成腈[2]。該反應需要使用過量的堿,常用的堿為t-BuOK。反應一般在室溫下數小時內完成,產率中等到較高水平 (式1,式2)[3,4]。TosMIC與甾體17-酮反應時,生成兩種異構體的混合產物 (式3)[5]。

TosMIC 與鹵代烴發生烷基化反應,生成多一節碳的酮[6,7]。分子間的烷基化反應生成對稱或者不對稱的鏈狀酮,分子內的烷基化反應生成相應的環狀酮 (式4,式5)[6,7]。

TosMIC 與α,β-不飽和羰基化合物反應生成吡咯環的反應一般給出較好的產率,羰基或者其它拉電子基團的存在對反應活性的影響非常大 (式6,式7)[8,9]。最近有人報道簡單的芳基乙烯就可以與TosMIC 發生形成吡咯環的反應 (式8)[10]。

酮轉化為氰基示例

To a 250 mL round-bottomed flask equipped with condenser and nitrogen inlet were added 4.34 g (23.49 mmol) N-carboethoxyperhydroazepin-4-one (prepared according to the procedure given by Z. G. Finney and T. N. Riley, J. Med. Chem., 23, 895, 1980), 10.53 g(54.02 mmol) tosylmethylisocyanide and 117 mL 1,2-dimethoxyethane. The solution was cooled to 0 deg C and 2.48 mL (54.02 mmol) ethanol and 9.21 g (82.2 mmol) potassium t-butoxide were added. The mixture was heated at 60 deg C for 18 hours, cooled and concentrated. The residue was taken up in ethyl acetate, washed with brine, dried over sodium sulfate and concentrated to give an oil. The oil was purified by chromatography on silica gel using hexane/ethyl acetate as eluent to afford 4.6 g (100percent) of oil.
用2,4,6- 三異丙基磺酰肼-KCN 將酮轉化為氰基

3-Quinuclidinone (24.2 g, 0.19 mol) and 2,4,6-triisopropylbenzenesulphonohydrazide (72g, 0.24 mol) were stirred together in anhydrous MeOH (250 mL) for 3 h. Potassium cyanide (33.8 g, 0.51 mol) was added and the mixture was heated under reflux for 5 h. The residue after evaporation of the solvent was partitioned between water and CH2C12. The organic phase was dried and evaporated and the residue was fractionally distilled under reduced pressure to give 3-cyanoquinuclidine (32, 6.1 g).