醛和酮還原轉變成醇的方法一直是分別制備伯醇和仲醇的一個較為常用的方法。還 原醛和酮到醇主要方法可以分為兩類:化學還原法和催化氫化法。
1、醛和酮的加氫還原
醛和酮的羰基也可通過加氫還原為相應的伯醇和仲醇。催化劑一般為負載在載體上的鎳、鉻、銅或銅鉻等。若加氫原料含有硫化物,則采用鎳、鈷或鎢的氧化物或硫化物 型抗硫催化劑。
醛和酮的氫化活性通常大于芳環而小于不飽和鍵。醛比酮更易氫化,所以醛加氫條 件比較溫和,一般溫度為 50~150℃(采用鎳或鉻催化劑)或 200~250℃(采用硫化物 催化劑);而酮加氫的條件在采用上述相同的催化劑時則相應地溫度為 150~250℃及 300~350℃。為了加速反應,及提高平衡反應的轉移,這類化合物加氫時也在壓力下進 行。用鎳催化劑時,壓力為 1~2Mpa,鉻催化劑時為 5~20Mpa,而用硫化物催化劑時 則為 30Mpa。 脂肪族醛,酮的氫化活性較之芳香族醛,酮為低,通常用 Raney 鎳和鉑為催化劑,而鈀催化劑的效果較差。一般需在較高溫度和壓力下還原。例如,由葡萄糖氫化得山梨 醇(sorbitol)。
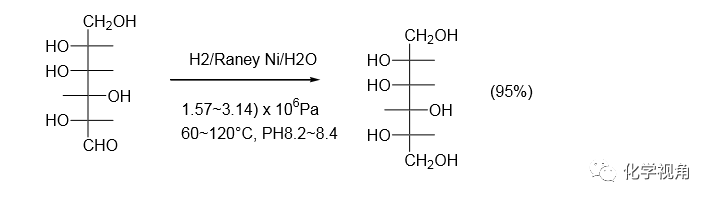
芳香醛酮的氫化還原,如用鈀為催化劑,在加氫劇烈時生成的醇會進一步氫解為烴特別對于芳環上有強推電子基團存在或二芳基酮(。但如選用適當活 性的 Raney 鎳為催化劑,在溫和的條件下,可得到醇。如天麻素(gastrodine)中間體的 制備。
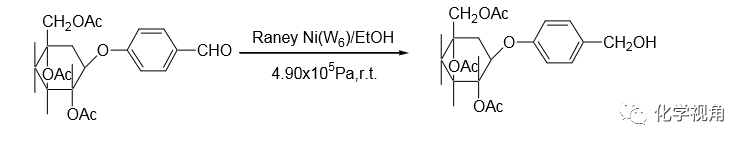 催化劑釕,銠,銥等金屬的三苯膦等配合物,在強堿條件下,可成功地還原脂肪酮 和芳香酮為相應的醇
催化劑釕,銠,銥等金屬的三苯膦等配合物,在強堿條件下,可成功地還原脂肪酮 和芳香酮為相應的醇
醛和酮的加氫還原示例
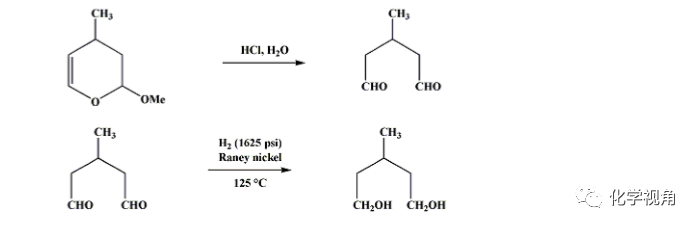
In a 2-l. three-necked flask equipped with a stirrer and thermometer are placed 336 g. (2.62 moles) of 3,4-dihydro-2-methoxy-4-methyl-2H-pyran, 630 ml. of water, and 24 ml. of concentrated hydrochloric acid. The mixture is stirred for 2 hours, during which the temperature may reach 50℃ but should not be permitted to rise higher. Solid sodium bicarbonate is then added until the solution is neutral to pH indicator paper. The entire reaction mixture weighing about 1 kg. together with 39 g. of Raney nickel2 is introduced into a 3-l. stainless-steel rocking hydrogenation autoclave. A hydrogen pressure of at least 1625 p.s.i. is applied, and the autoclave is heated to 125℃ and held there with shaking for 4 hours. The mixture is allowed to cool overnight, and the catalyst is separated either by suction filtration through Filter-Cel or by centrifugation. The solution is distilled through a 12-in. Vigreux column. After the methanol and water are separated, the 3-methyl-1,5-pentanediol distils at 139–146℃/17 mm., 149–150℃/25 mm. The yield is 251–256 g. (81–83%), n25D 1.4512–1.4521.