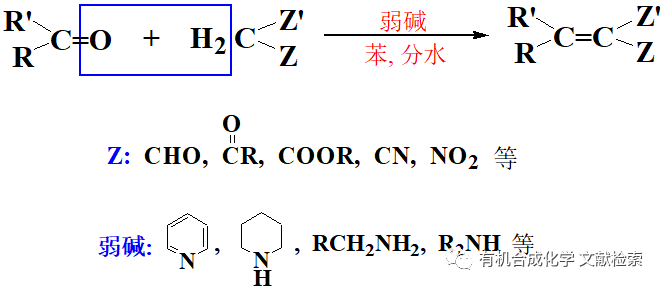
由二鹵甲基或二鹵亞甲基合成醛酮
將二鹵甲基或二鹵亞甲基化合物在酸性或堿性條件下水解,則生成相應的醛酮。比如 9,9-二溴芴在醋酸中,于醋酸鈉存在下加熱回流即可以良好的收率生成芴酮。


p-Clorobenzal cloride was added to a 4-l. wide-mouthed bottle containing 400 cc. of concentrated sulfuric acid, and stirred vigorously (Hood) for five hours. The viscous mixture is then transferred to a separatory funnel and allowed to stand overnight, after which the lower layer is run slowly, with stirring, into a 3-l. beaker three-quarters filled with cracked ice. The cream-colored solid obtained when the ice has melted is filtered by suction, washed with water, pressed dry on the funnel, and divided into three equal parts. Each portion is dissolved in a minimum of ether, and the ether solution is repeatedly shaken with 2 per cent sodium hydroxide solution until acidification of the washings gives no precipitate of p-chlorobenzoic acid. After removal of the ether by distillation on a steam bath, the residue is distilled under diminished pressure from a Claisen flask. The yield of p-chlorobenzaldehyde distilling at 108–111°/25 mm. and melting at 46–47° is 76–84 g. (54–60 per cent of the theoretical amount).
c將鹵化物變為鎂(Grignard 試劑)或鋰化物等,進行酰化可以合成醛酮。由鎂(Grignard 試劑)或鋰化物等進行酮的合成將在由羧酸極其衍{attr}2168{/attr}醛酮部分詳細討論,本部分只對有機金屬化合物的甲酰化合成醛作一全面闡述。常用的甲酰化試劑有:FCHO,(HCO)2O HCOOCOCH 3 ,CH(OCH3)3 ,HCO2C2H5 ,HCO2Li PhN=CHOC2H5 ,Ph-N(CH3 )-CHO,DMF,LM(CO)x, 由有機金屬化合物合成醛的甲酰化試劑以甲酸酯類、甲酰胺類用得較普遍,以 DMF 或N-甲酰哌啶使用較為方便。有機金屬化合物(有機鎂、有機鋰化合物)與過量原甲酸脂的反應廣泛用于脂醛及芳醛的合成,產率達 55%-90%。除原甲酸脂外,甲酰胺、乙氧亞甲基苯胺(ethoxymethyleneaniline,C6H5N=CHOC2H5)也為常用的甲酰化試劑,其中以甲酰胺{attr}2239{/attr}最為常見,常稱為 Bouveault 反應。 合成反應示例

Preparation of 4-Fluoro-2-methylbenzaldehyde

n-BuLi (2.6M in hexane, 57 ml, 143 mmol) was added over 15 minutes to a THF solution(200 ml) of 2-methyl-4-fluorophenylbromide (24.5 g, 130 mmol), cooled to -78.deg. C. and allowed to stir 1 hour at -78.deg. C. DMF (26.61 gm, 364 mmol) was then added over 2 minutes, and the solution was allowed to stir another hour. The reaction was quenched with NH4Cl and warmed to room temperature. 10% HCl was added until the solution became acidic. The mixture was diluted with ether and the organic layer washed with water and brine, then dried over MgSO 4 , filtered and concentrated to give an orange oil. The oil was purified by distillation (bp=69℃,at 7 mm Hg) to afford aldehyde 4-fluoro-2-methylbenzaldehyde as a clear liquid (13.3 g, 74%). TLC: Rf 0.30 (10% EtOAc in hexane)。
由 Pd 催化反應合成醛 芳基鹵化物及乙烯基鹵化物在 Pd 催化劑的存在下與 H 2 及 CO 反應則生成醛。 該法為高壓反應,如用 Si-H 或 Sn-H 為還原劑,則也可在低壓下進行甲酰化。